
What Upneeq
®
(oxymetazoline hydrochloride
ophthalmic solution), 0.1%
Offers Your Patients
ophthalmic solution), 0.1%
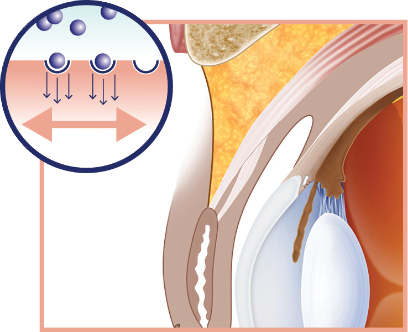
Upneeq is a first-in-class treatment—with a mechanism of action that lifts
upper eyelids
- Directly activates α-adrenergic receptors in Müller’s muscle, stimulating contraction and elevation of the upper eyelids in adults with low-lying lids 1
-
In vitro functional assays demonstrate that oxymetazoline has ≈5-fold greater affinity for α2 receptors 2,3 than for
α1 receptors 2,3- The pharmacology of Upneeq has not been fully elucidated with respect to pharmacodynamic contribution of each receptor subtype

Upneeq stimulates Müller’s muscle to raise the upper eyelid 1
Delivers rapid, visible lift to the upper eyelid with once-daily use
Mean change from baseline in upper eyelid elevation (MRD-1) after dosing Upneeq (data from pooled analysis of 2 clinical trials) *4
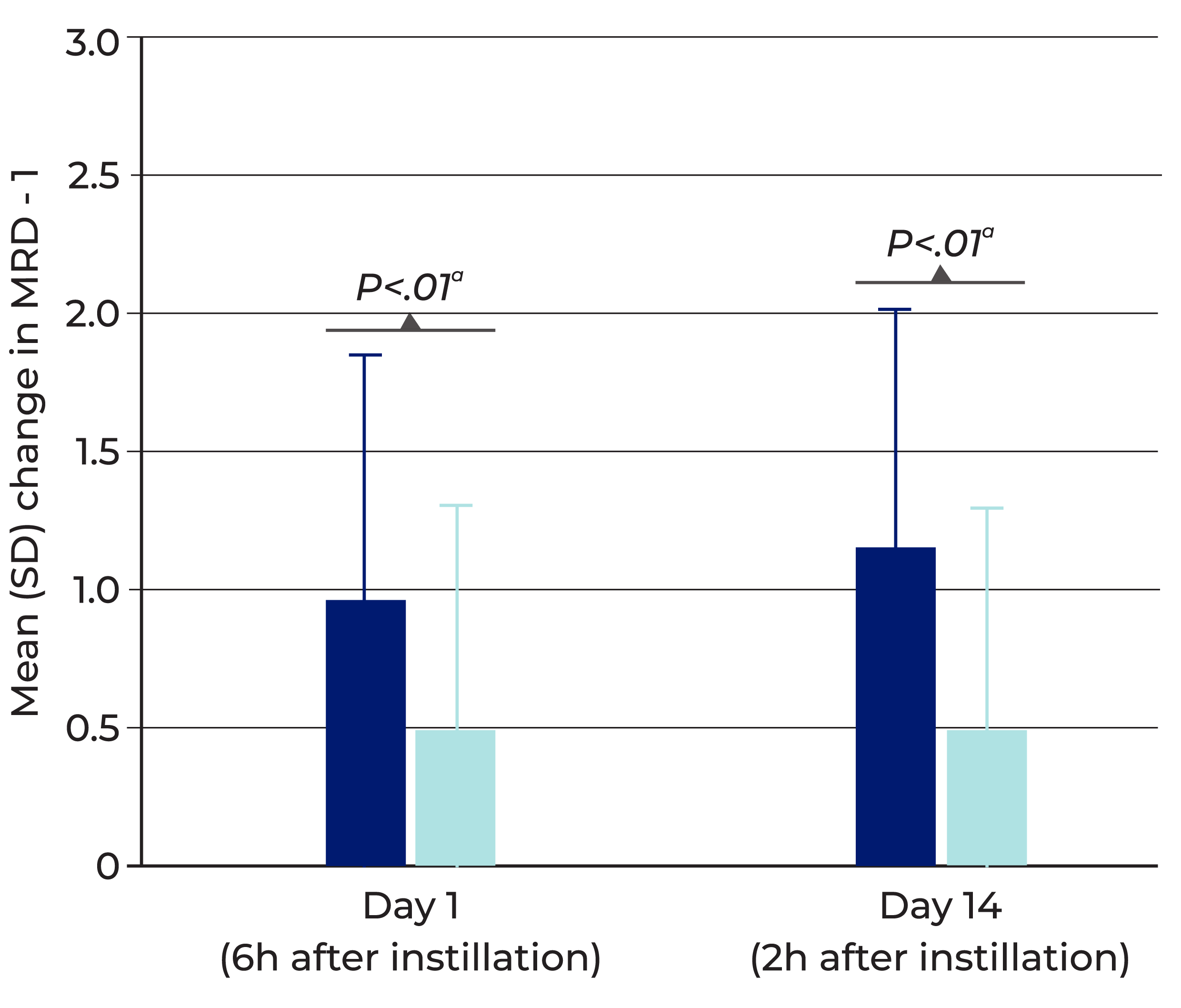
Error bars represent standard deviation of the mean change from baseline.
-
Upneeq increased upper eyelid elevation significantly more than vehicle in clinical trials, as measured by MRD-1 (on Day 1 and Day 14 of treatment)
1
- Achieved average eyelid lift of 1mm 5
-
In one clinical trial, onset of elevation as shown by improvement in MRD-1 began in some patients 5 minutes after Upneeq was administered (the earliest time point measured)
5
- RVL-1201-202, Upneeq (n=109) vs. vehicle (n=55)
MRD-1=Marginal Reflex Distance 1 (distance from the central pupillary light reflex to the central margin of the upper lid).

Examples of positive results after the first dose of Upneeq
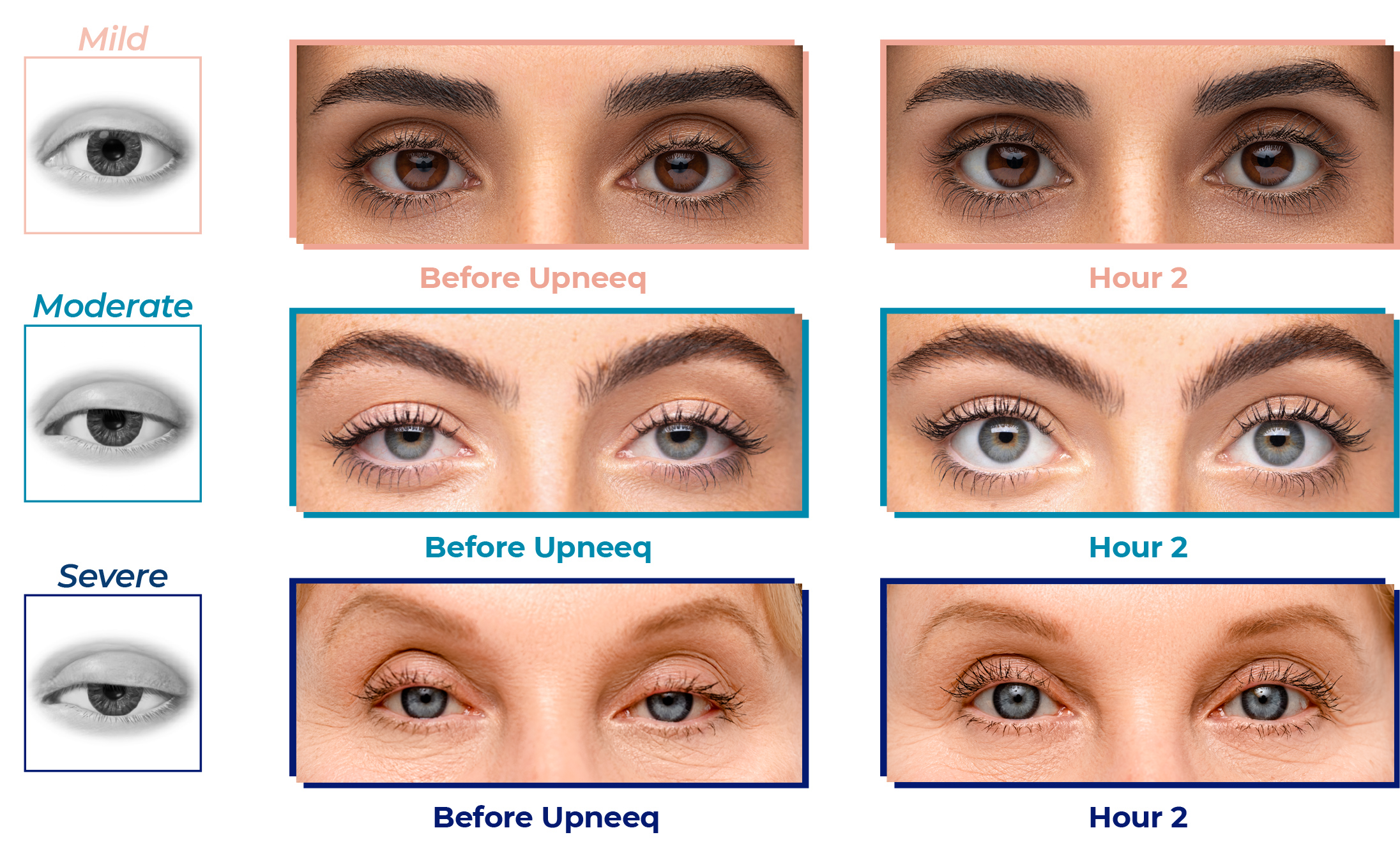
Actual patient images courtesy of Dr. Robert Schwarcz and Dr. Raymond Douglas. Individual results may vary. Average upper eyelid lift with Upneeq in clinical trials was 1mm.* 5
Achieves significant visual field improvement
Change from baseline in points seen in top 4 rows on LPFT (data from pooled analysis of 2 clinical trials)* 4
Error bars represent standard deviation of the mean change from baseline.
- Upneeq demonstrated 3 times greater improvement in visual field compared to vehicle in clinical trials ( P<.01), as measured by LPFT (on Day 1 and Day 14 of treatment) 1
LPFT=Leicester Peripheral Field Test (measuring superior visual field).
*Two randomized, multicenter, double-masked, vehicle-controlled, Phase 3 studies compared once-daily Upneeq in 304 subjects with acquired blepharoptosis. Efficacy was assessed with the Leicester Peripheral Field Test (LPFT) and photographic measurement of Marginal Reflex Distance (MRD-1). There was a statistically significant difference in mean change in the LPFT from baseline after instillation of Upneeq and vehicle, with significantly greater increases in the study eye of the Upneeq group evident at the 2-hour point and maintained at the 6-hour time point. Greater MRD-1 increases were observed for the Upneeq group than the vehicle group on Day 1 at 6 hours post-dose and on Day 14 at 2 hours post-dose. 1
Safety and tolerability
In clinical trials, Upneeq safety was comparable to placebo
- Most common adverse reactions (incidence 1%-5%): punctate keratitis, conjunctival hyperemia, dry eye, blurred vision, instillation site pain, eye irritation and headache 1
- 2.2% of patients in clinical trials discontinued treatment due to an adverse event 5
Preservative-free and formulated with hypromellose
- Hypromellose is a vehicle commonly used in artificial tear solutions and is comfortable for most patients 6,7
High completion rate in clinical trials
- 97.5% of subjects completed Upneeq clinical trials 4
- 98% of subjects were compliant with dosing Upneeq once daily 4
- 94% of patients indicated no discomfort with Upneeq 5





